

Trying to keep your blood sugar in range can be stressful. Control‑IQ technology makes it easier by using CGM values* to predict glucose levels 30 minutes ahead and automatically adjusting insulin delivery.
![]()
Control-IQ technology increases basal insulin dosing and delivers automatic correction boluses † (up to one an hour) if sensor glucose is predicted to be high.
![]()
Control-IQ technology can decrease or stop basal insulin if sensor glucose is predicted to be low. This can help improve time in range overnight and during the day.
![]()
Icons indicate whether insulin dosing is increasing, decreasing, or being stopped by Control-IQ technology.
See How it Works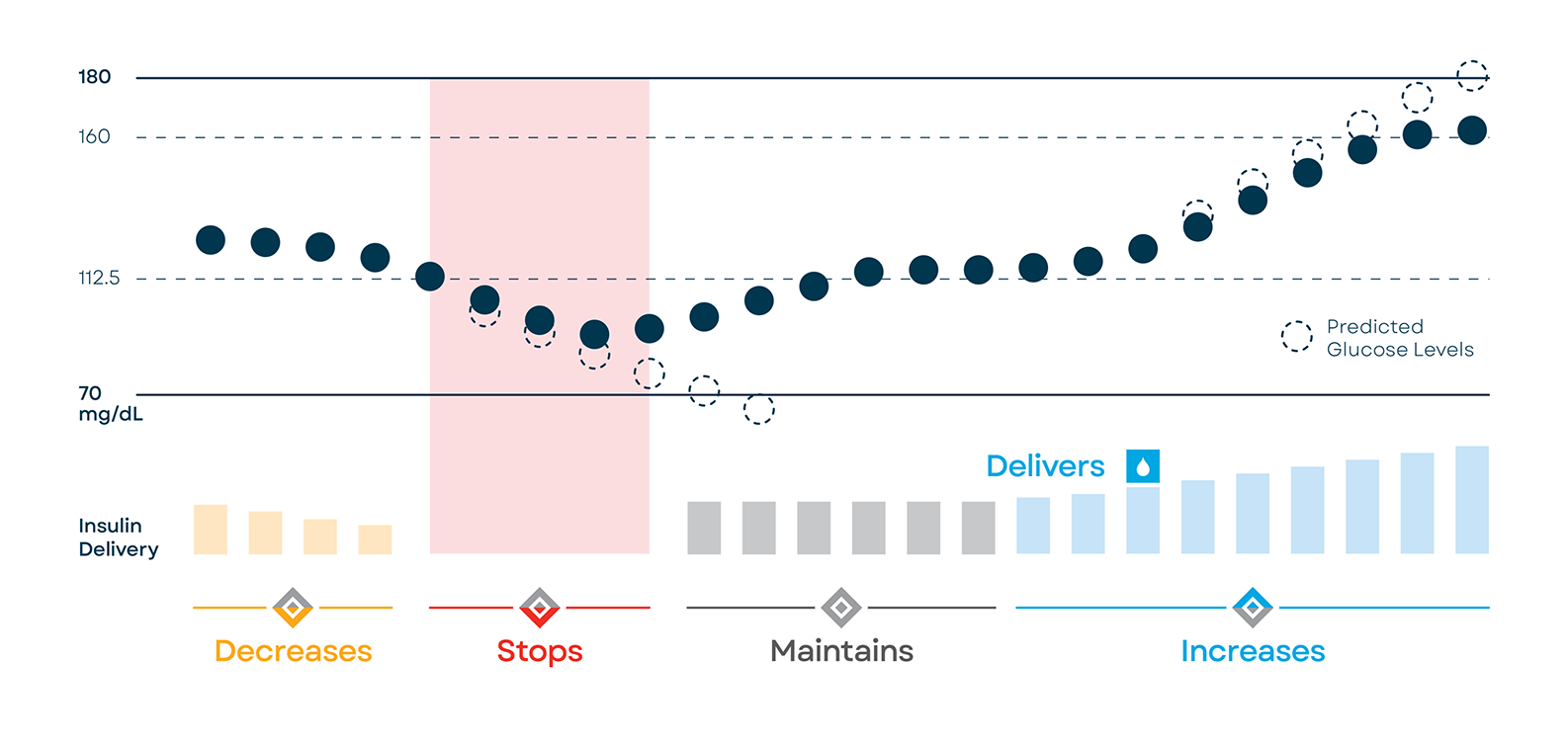

![]()
See how Tandem automated insulin delivery systems are changing lives for the better by significantly improving time in range and reducing the burden of diabetes management. 2
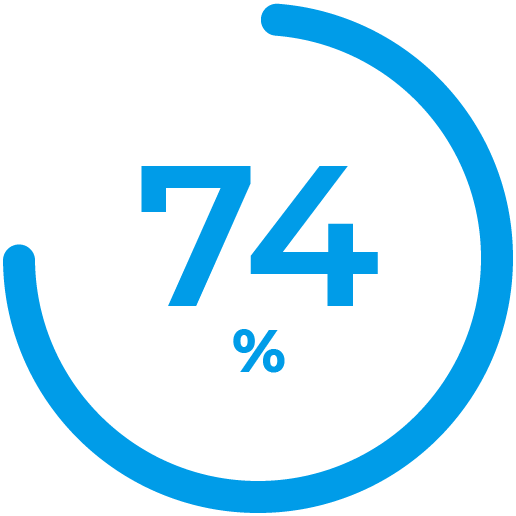
Average time in range per day for real-world users of Control-IQ technology after 12 months. 3
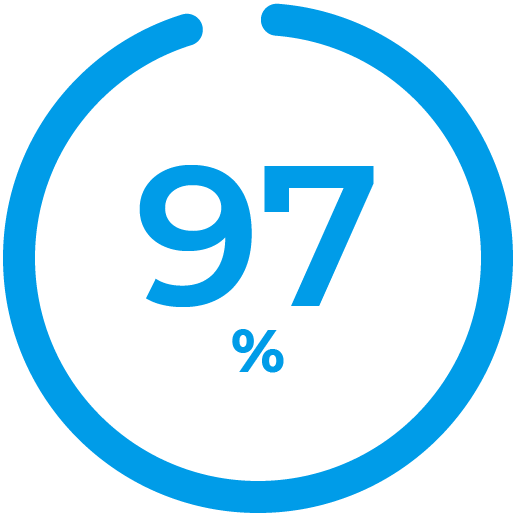
Said it’s Easy to Use
Percent of clinical study participants who used Control-IQ technology and said it was easy to use. 4
Control-IQ technology includes optional activity settings that adjust the range of treatment values.
![]()
Uses a narrower and higher range of treatment values to help guard against lows during activity.
![]()
Uses a narrower and lower range of treatment values to help guard against lows and highs while sleeping.

![]()
Tandem Diabetes Care is the #1 recommended insulin pump brand by people living with diabetes — four years and counting! 5 Choose which Tandem automated insulin delivery system best fits your lifestyle.


![]()
Test drive the easy-to-use interfaces of either Tandem Mobi or t:slim X2 automated insulin delivery systems, with no obligation.
![]()
See what people are saying about the t:slim X2 insulin pump and how it is changing their lives for the better.
![]()
Choose from a variety of cannula materials, tubing lengths, and insertion angles to fit your needs.
![]()
Our mobile and cloud-based applications are designed to help you better manage your diabetes.
Whether you're ready to get a Tandem insulin pump, or looking to check insurance coverage, you’ll find everything you need here.
Responsible Use of Control‑IQ Technology
Even with advanced systems such as Control-IQ technology, you are still responsible for actively managing your diabetes. Control-IQ technology does not prevent all high and low blood glucose events. The system is designed to help reduce glucose variability, but it requires your accurate input of information, such as meals and periods of sleep or exercise. Control-IQ technology will not function as intended unless you use all system components, including your CGM, infusion sets and pump cartridges, as instructed. Importantly, the system cannot adjust your insulin dosing if the pump is not receiving CGM readings. Because there are situations and emergencies that the system may not be capable of identifying or addressing, always pay attention to your symptoms and treat according to your healthcare provider’s recommendations.
* CGM sold separately.
† If glucose values are predicted to be above 180 mg/dL, Control-IQ technology calculates a correction bolus using the Personal Profile settings and a target of 110 mg/dL and delivers 60% of that value.
1. Beck RW, Kanapka LG, Breton MD, et al. A Meta-Analysis of Randomized Trial Outcomes for the t:slim X2 Insulin Pump with Control-IQ technology in Youth and Adults from Age 2 to 72. Diabetes Technol Ther. 2023;25(5):329-342. doi: 10.1089/dia.2022.0558 4
2. Pinsker JE, Singh H, Graham R, et al. Significant Reductions in Adverse Events and Hospitalizations with Control-IQ Technology in Pediatric Users with Type 1 Diabetes: Results from the CLIO Study. Poster presented at International Society for Pediatric and Adolescent Diabetes 47th Annual Conference; October 13-1S, 2021; Virtual.
3. Breton MD, Kovatchev BP. One year real-world use of the Control-IQ advanced hybrid closed-loop technology. Diabetes Technol Ther. 2021;23(9):601-608. doi: 10.1089/dia.2021.0097.
4. Kudva YC, Laffel LM, Brown SA, et al. Patient-Reported Outcomes in a Randomized Trial of Closed-Loop Control: The Pivotal International Diabetes Closed-Loop Trial. Diabetes Technol Ther. 2021;23(10):673-683. doi: 10.1089/dia.2021.0089.
5. dQ&A US Diabetes Connections Patient Panel Report; Net Promoter Score; Jan. 2019-Sept. 2023: P.49, Sept. 2023.
Important Safety Information
Indications for Use
Tandem Mobi system: The Tandem Mobi insulin pump with interoperable technology (the pump) is intended for the subcutaneous delivery of insulin, at set and variable rates, for the management of diabetes mellitus in persons requiring insulin. The pump is able to reliably and securely communicate with compatible, digitally connected devices, including automated insulin dosing software, to receive, execute, and confirm commands from these devices. The pump is intended for single patient, home use and requires a prescription. The pump is indicated for use in individuals 6 years of age and greater.
t:slim X2 insulin pump: The t:slim X2 insulin pump with interoperable technology is intended for the subcutaneous delivery of insulin, at set and variable rates, for the management of diabetes mellitus in people requiring insulin. The pump is able to reliably and securely communicate with compatible, digitally connected devices, including automated insulin dosing software, to receive, execute, and confirm commands from these devices. The pump is intended for single patient use. The pump is indicated for use with NovoLog or Humalog U-100 insulin. The pump is indicated for use in individuals 6 years of age and greater.
Control-IQ technology: Control-IQ technology is intended for use with compatible integrated continuous glucose monitors (iCGM, sold separately) and alternate controller enabled (ACE) pumps to automatically increase, decrease, and suspend delivery of basal insulin based on iCGM readings and predicted glucose values. It can also deliver correction boluses when the glucose value is predicted to exceed a predefined threshold. Control-IQ technology is intended for the management of Type 1 diabetes mellitus in persons 6 years of age and greater. Control-IQ technology is intended for single patient use. Control-IQ technology is indicated for use with NovoLog or Humalog U-100 insulin.
Warning: Control-IQ technology should not be used by anyone under the age of 6 years old. It should also not be used in patients who require less than 10 units of insulin per day or who weigh less than 55 pounds.
Control-IQ technology is not indicated for use in pregnant women, people on dialysis, or critically ill patients. Do not use Control-IQ technology if using hydroxyurea. Users of a Tandem insulin pump and Control-IQ technology must use the insulin pump, CGM, and all other system components in accordance with their respective instructions for use; test blood glucose levels as recommended by their healthcare provider; demonstrate adequate carb-counting skills; maintain sufficient diabetes self-care skills; see healthcare provider(s) regularly; and have adequate vision and/or hearing to recognize all functions of the pump, including alerts, alarms, and reminders. The Tandem pump must be removed before MRI, CT, or diathermy treatment. Visit tandemdiabetes.com/safetyinfo for additional important safety information.
Back to top